Body-heat activated shape memory polymer advances minimally invasive stent design
FAYETTEVILLE, GA, UNITED STATES, November 15, 2025 /EINPresswire.com/ -- Minimally invasive medical implants require materials that can be delivered in compact form and recover their functional shape once inside the body. However, many current shape memory polymers either demand high activation temperatures or lack sufficient mechanical force for vascular use. This study introduces a micelle-enhanced shape memory polymer that combines digital light processing (DLP) 4D printing with dual responsiveness to body heat and aqueous swelling. The printed structures maintain mechanical strength, biocompatibility, and strong recovery force, allowing them to expand under mild physiological conditions, such as 37 °C and normal hydration levels. This approach offers a promising platform for patient-specific and self-deploying vascular implants.
Vascular implants, including stents, are widely used to reopen blocked or narrowed vessels and restore blood flow. Conventional stents are often constructed from metals or thermally activated polymers, which may complicate deployment due to rigid geometry, high actuation temperatures, or suboptimal mechanical match with soft tissues. Additive manufacturing has enabled customizable device designs, yet achieving reliable shape recovery at body-safe conditions while generating sufficient radial expansion force remains challenging. Additionally, stents must maintain biocompatibility and mechanical durability under continuous dynamic loading. Based on these challenges, it is necessary to develop a clinically adaptable shape memory material capable of gentle, precise, and strong recovery under physiological stimulation.
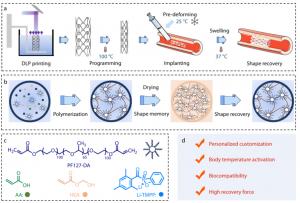
Researchers from the State Key Laboratory of Flexible Electronics at Northwestern Polytechnical University have developed a dual-stimuli responsive shape memory polymer that can be 4D-printed into customized vascular implants. The work was published (DOI: 10.1007/s10118-025-3423-6) in Chinese Journal of Polymer Science in 2025. The polymer is enhanced by self-assembled PF127-DA micelles, enabling activation by the body’s natural temperature and fluid environment, allowing expansion without external heating or irradiation. This innovation advances minimally invasive deployment and patient-specific implant design.
The material is produced using digital light processing (DLP)-based 4D printing, enabling high-precision fabrication of complex geometries. Within the precursor ink, PF127-DA amphiphilic molecules self-assemble into nanoscale micelles that function as structural crosslinkers. After printing and drying, the resulting polymer exhibits strong mechanical robustness and stable shape fixation. When exposed to physiological conditions—37°C and aqueous media—the polymer undergoes rapid shape recovery driven by both thermal transition and swelling-induced expansion. Mechanical testing demonstrated recovery stress reaching ~150 kPa, significantly higher than thermally responsive polymers alone. In vitro biocompatibility assays with NIH/3T3 fibroblasts confirmed low cytotoxicity and favorable cellular adhesion. A vascular occlusion model further verified functional deployment: a compressed stent expanded from 8 mm to 16 mm diameter under body-temperature PBS, reopening the simulated vessel lumen. These results collectively validate its ability to combine high expansion force with tissue-matching softness for minimally invasive implantation.
“The micelle-enhanced network allows the polymer to react naturally to the physiological environment,” said the corresponding author. “By enabling strong yet gentle expansion at body temperature, the material provides both deployment convenience and compatibility with soft vascular tissues. The platform also benefits from the geometric freedom of 4D printing, allowing each implant to be uniquely tailored to patient anatomy.”
This micelle-enhanced 4D printing system offers a new strategy for designing minimally invasive vascular implants and could be extended to soft tissue scaffolds, drug-release devices, and adaptive biointerfaces. Its ability to combine shape programmability, strong recovery force, and mild activation makes it particularly suitable for patient-specific therapies. Future work may explore biodegradable versions and in vivo validation, potentially advancing customizable stent therapy and broader soft-implant applications in regenerative medicine.
References
DOI
10.1007/s10118-025-3423-6
Original Source URL
https://doi.org/10.1007/s10118-025-3423-6
Funding Information
B.Z. acknowledges Natural Science Basic Research Program of Shaanxi (No. 2025JC-YBMS-358) and the Fundamental Research Funds for the Central Universities (No. D5000250307).
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.